Details of the Drug
General Information of Drug (ID: DMXJ7O8)
| Drug Name |
Vitamin C
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
l-ascorbic acid; vitamin C; 50-81-7; L(+)-Ascorbic acid; Ascorbicap; Cevitamic acid; Ascoltin; Hybrin; Secorbate; Proscorbin; Lemascorb; Ascorteal; Ascorbajen; Vitascorbol; Vitamisin; Natrascorb; Citriscorb; Cescorbat; Ascorbutina; Testascorbic; Cevitamin; Cetemican; Allercorb; Vitacimin; Roscorbic; Laroscorbine; Viscorin; Redoxon; Cenetone; Cegiolan; Cebicure; Ascorvit; Viforcit; Concemin; Cevital; Cergona; Cemagyl; Cebione; Cantaxin; Ascorin; Vitacee; Vicelat; Cevimin; Cetamid; Cenolate; Celaskon; Vitacin; Adenex; Ascorb; Ascorbicab; Ascorbicin; Ascorbinsaeure; Cantan; Cebid; Cebion; Cecon; Ceglion; Celin; Cemill; Cereon; Cetebe; Cevalin; Cevatine; Cevex; Cevibid; Cevitan; Cevitex; Cewin; Ciamin; Cipca; Colascor; Cortalex; Duoscorb; Ferancee; Hicee; Magnorbin; Ribena; Scorbacid; Semidehydroascorbate; Sodascorbate; Stuartinic; Sunkist; Tolfrinic; Vitace; Xitix; Acid Ascorbic; Acide ascorbique; Acido ascorbico; Acidum ascorbicum; Acidum ascorbinicum; Antiscorbic vita min; Antiscorbic vitamin; Antiscorbutic factor; Antiscorbutic vitamin; Ascorbyl radical; Catavin C; Ce lent; Component of Cortalex; Component of Endoglobin Forte; Component of Ferancee; Davitamon C; Ferrous ascorbate; Kyselina askorbova; Kyselina askorbova [Czech]; Magnesium Ascorbicum; Monodehydroascorbic acid; Natrascorb injectable; Oral Vitamin C; Planavit C; Roscorbi c; Vicomin C; Acide ascorbique [INN-French]; Acido ascorbico [INN-Spanish]; Acidum ascorbicum [INN-Latin]; Arco-cee; Ascoltin (TN); Ascorbic Acid, Monosodium Salt; Ascorbicap (TN); C-Level; C-Long; C-Quin; C-Span; C-Vimin; Cee-Caps TD; Cee-Vite; Cee-caps td; Cetane-Caps TC; Cetane-Caps TD; Cevi-Bid; Component of E and C-Level; IDO-C; Iron(II) ascorbate; Iron-ascorbic acid complexes; L-Ascorbic acid; L-Lyxoascorbic acid; L-Xyloascorbic acid; L-ascorbic acid; Liqui-Cee; Meri-C; Sodium Ascorbate (Ascorbic Acid); Vitamin-C; Ascorbic acid [BAN:INN:JAN]; Ascorbic acid [INN:BAN:JAN]; CE-VI-Sol; Ce-Mi-Lin; Dora-C-500; L-3-Ketothreohexuronic acid lactone; L-3-ketothreohexuronic acid; L-Ascorbic acid, free radical form; Ascor-bid; Ascorbic acid (JP15/USP/INN); Ascor-BID; L-(+)-Ascorbic Acid; L-threo-Hex-2-enonic acid, gamma-lactone; L-threo-hex-1-eofuranos-3-ulose; L-threo-hex-2-enono-1,4-lactone; (2R)-2-[(1S)-1,2-Dihydroxyethyl]-4,5-dihydroxy-furan-3-one; (2R)-2-[(1S)-1,2-dihydroxyethyl]-4,5-dihydroxyfuran-3-one; (5R)-5-[(1S)-1,2-dihydroxyethyl]-3,4-dihydroxyfuran-2(5H)-one; 2-(1,2-Dihydroxyethyl)-4,5-dihydroxyfuran-3-one; 3-Keto-L-gulofuranolactone; 3-Oxo-L-gulofuranolactone; 3-Oxo-L-gulofuranolactone (enol form); [14C]ascorbic acid
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Vitamins
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
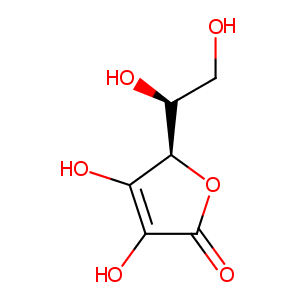 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 176.12 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1.6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Vitamin C (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
